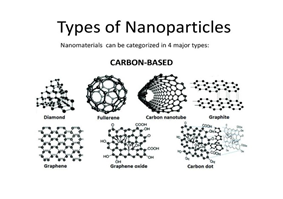
Carbon nanomaterials have gotten plenty of interest in recent years due to their unique properties such as high surface-to-volume ratio, excellent electron transfer rate, high mechanical strength, and strong conductive properties (Seekaew et al., 2014). They are also very sensitive at room temperature and have minimal sensing material requirements which allowed the researchers to provide a low-power and low-cost gas sensor (Mittal & Kumar, 2014). Additionally, recent research in nanomaterial technology found that carbon nanomaterials have great potential in replacing gas sensors using metal oxide materials.
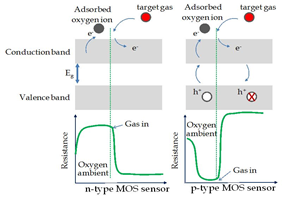
The gas sensor technology is incredibly important today to detect toxic gas present in our atmosphere. Generally, carbon nanomaterials are behaving like p-type semiconductors with hole-like charge carriers. Therefore, when a carbon-based gas sensor is subjected to a high concentration of toxic gas (reducing gas), holes within the valence band of carbon nanomaterials are depleted, leading to increased device resistance (Seekaew et al., 2014). This is due to that trapped on the surface of the carbon sensing film before the exposure reacts with the reducing gas and releases the electrons to carbon. The electrons within the conduction band recombine with holes from the valence band resulting in a low charge carrier concentration and high resistance (Seekaew et al., 2014).
The change in resistance depends on the rate of adsorption and desorption of toxic gas molecules into the sensing film as well as the type of target gas. The target gas is either an oxidizing gas or a reducing gas. Modification of carbon nanomaterials structure by adding functional group can improve its reactivity and sensitivity to adsorb the target gas faster. The most common functional groups are carboxyl, hydroxyl, carbonyl, and amino (Mittal & Kumar, 2014).
References
- Mittal M., & Kumar A. (2014). Carbon Nanotube (CNT) Gas Sensors for Emissions from Fossil Fuel Burning, Sensors and Actuators B: Chemical. Retrieved from http://dx.doi.org/10.1016/j.snb.2014.05.080
- Seekaew, Y., Lokavee, S., Phokharatkul, D., Wisitsoraat, A., Kerdcharoen, T., & Wongchoosuk, C. (2014). Low-cost and flexible printed graphene–PEDOT:PSS gas sensor for ammonia detection. Organic Electronics, 15(11), 2971–2981. Retrieved from https://doi.org/10.1016/j.orgel.2014.08.044
By: Farihah Abd Rahman, Internship Student, Functional Devices Laboratory, Institute of Advanced Technology (ITMA), UPM.
For further information, please contact:
Dr. Intan Helina Hasan
Research Officer
Functional Devices Laboratory (FDL)
Institute of Advanced Technology, UPM
i_helina@upm.edu.my
Date of Input: 08/10/2021 | Updated: 10/08/2023 | roslina_ar
MEDIA SHARING