Conventional anti-cancer drugs, like doxorubicin, often exhibit high toxicity and indiscriminate distribution in the body, leading to various side effects. Cancer cells can leverage autophagy to withstand stress induced by these drugs, contributing to drug resistance in advanced tumors. Targeting pro-apoptotic survival pathways, such as autophagy, could enhance the effectiveness of existing therapies at lower concentrations, potentially minimizing side effects. SAR405, an autophagy inhibitor, faces challenges like extracellular premature degradation and poor cellular uptake. On the other hand, chitosan nanoparticles are recognized as biodegradable, non-toxic, and biocompatible drug delivery agents that can reduce side effects while improving intracellular drug uptake. This study introduces a dual approach to enhance doxorubicin efficacy and inhibit autophagy using a nano-mediated delivery system. Autophagic inhibition is achieved by enhancing SAR405 cellular uptake through SAR405-loaded chitosan nanoparticles. The synthesized nanoparticles are then characterized for hydrodynamic diameter, polydispersity, encapsulation, and drug loading efficiencies.
The nanoparticles underwent morphological characterization through electron microscopy, and the combined effects of SAR405-loaded chitosan nanoparticles with doxorubicin were evaluated for cytotoxicity using MTT and Annexin-V apoptosis assays. Autophagy progression, as indicated by autophagosome formation, was assessed using CYTO-ID staining. Following encapsulation, the size of SAR405-loaded chitosan nanoparticles increased significantly from 54 nm to 161 nm at a SAR405 concentration of 10 µM. The polydispersity index also rose from 0.11 to 0.31, indicating the presence of both encapsulated and unencapsulated components. Treatment of A549 lung cancer cells with doxorubicin at IC50 values, in combination with SAR405-encapsulated chitosan nanoparticles, resulted approximately 47% greater reduction in cell viability, as observed in the Annexin V-FITC/PI assay compared to doxorubicin alone. The nanoparticle-delivered SAR405 induced autophagy inhibition in the treated cells, representing the primary factor contributing to decreased cancer cell resistance to doxorubicin. This enhanced efficacy at lower concentrations suggests a promising approach to target cancer cell survival pathways, potentially improving the effectiveness of chemotherapeutic drugs.
This study employed a nanoparticle-mediated delivery system to enhance the intracellular uptake of SAR405 in A549 lung cancer cell models. Encapsulation within a delivery vector serves to shield the inhibitor from extracellular enzymatic degradation and packaging it into nano-sized dimensions may facilitate increased cellular uptake. Chitosan nanoparticles (CNPs) were chosen as the delivery system for SAR405, representing an inert polymeric nanoparticle system widely utilized in drug administration applications. In contrast to other nanomaterials that require surface modifications before in vivo and in vitro use, CNPs are a polymer-based organic nanomaterial that offers simpler preparation methods, biological compatibility, and improved in vivo distribution. The SAR405-encapsulated nanoparticle system was created by complexing the chitosan polymer (CS) with SAR405 and subsequently cross-linking with sodium tripolyphosphate (TPP), initiating particle formation through ionic gelation processes. (Fig. 1).
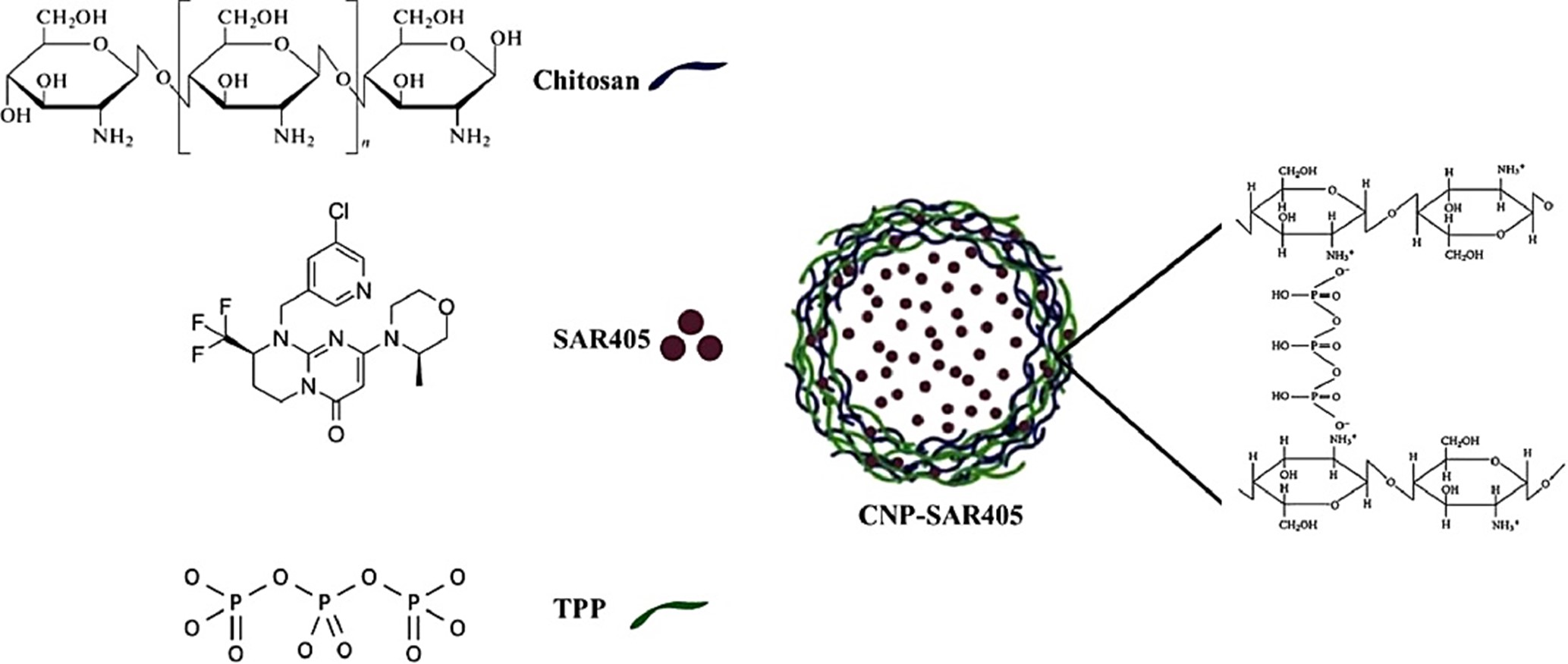
Fig. 1. A Schematic representation of the preparation of chitosan-TPP nanoparticles and SAR405-loaded chitosan.
The binary treatment approach involved utilizing the system with both doxorubicin and SAR405-encapsulated chitosan nanoparticles against A549 lung cancer cells. This combination induced apoptotic responses by simultaneously targeting cellular death through apoptotic pathways and sequentially inhibiting autophagy. By preventing cancer cells from activating autophagy as a pro-apoptotic response to apoptosis, the system effectively enhanced the anticancer efficacy of doxorubicin (see Fig. 2). As a result, comparable rates of cell death could be achieved at lower concentrations of doxorubicin, serving as a proof of concept that underscores the significance of adopting a multifaceted approach in future anticancer therapies.
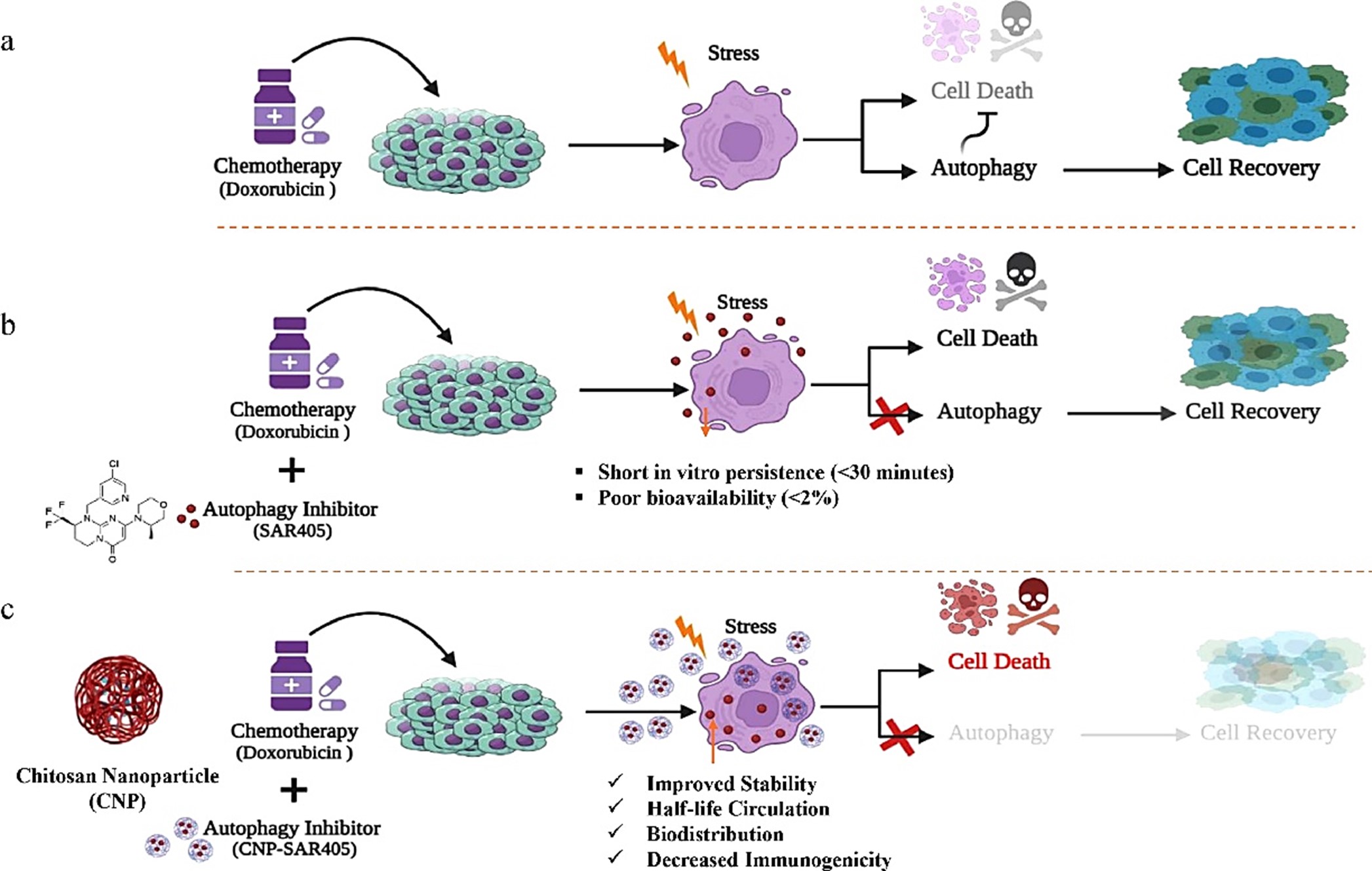
Fig. 2. A schematic representation of the binary treatment approach using CNPs as a delivery vector for SAR405. Doxorubicin, a potent cellular apoptotic agent may induce autophagic responses in cancer cells. As a result, this pro-apoptotic event leads to (a) cancer cell recovery and causes doxorubicin resistance in advanced cancers. Alternatively, autophagy inhibitors such as SAR405 may be able to prevent activation of autophagy but instead (b) suffers from limitations including short in vivo persistence as well as poor bioavailability. Therefore, (c) adoption of a nanoparticle delivery system such as CNPs increase the stability, intracellular uptake, and biodistribution of SAR405 in cells. Therefore, autophagy is arrested in the cancer cells, enhancing the efficacy of doxorubicin by inhibiting autophagy activation leading to increased cell death.
This study revealed that the CNP formulation exhibited a consistently spherical morphology, high monodispersed, and a size conducive to efficient intracellular delivery. The delivery of SAR405 by CNP not only heightened the effectiveness of SAR405 as an autophagy inhibitor but also enhanced the efficacy of doxorubicin as a chemotherapeutic drug. The CNP-SAR405 system further increase the efficiency of doxorubicin in inducing cell death by inhibiting the autophagy pathway, which cancer cells typically employ to resist chemotherapy drugs.
The findings from this research underscore the effectiveness of utilizing a nanoparticle system to enhance the efficiency of inhibitors. Moreover, it substantiates that the autophagy pathway serves as one of the mechanisms cancer cells use to resist chemotherapy drugs. Consequently, disrupting the autophagy pathway emerges as a promising strategy to augment the efficacy of chemotherapy drugs. In conclusion, this system holds potential for developing a novel cancer therapy featuring a "dual treatment" or "dual compounds" approach targeting different facets of cancer—autophagy and cytotoxicity. This approach has the added benefit of reducing side effects in cancer patients.
Source:
Mohammed Numan Alamassi, Suet Lin Chia, Che Azurahanim Che Abdullah, Mas Jaffri Masarudin. Increased efficacy of biologics following inhibition of autophagy in A549 lung cancer cells in bimodal treatment of doxorubicin and SAR405-loaded chitosan nanoparticles, OpenNano, Volume 11, 2023, 100142, https://doi.org/10.1016/j.onano.2023.100142 (https://www.sciencedirect.com/science/article/pii/S235295202300021X)
Date of Input: 26/12/2023 | Updated: 26/12/2023 | roslina_ar
MEDIA SHARING