Properties of nanomaterials
When nanoscale materials are decomposed, distinct changes in their properties can be seen. Quantum size effects lead to changes in the electronic properties of materials as they move from the molecular level to the nanoscale level. The mechanical, thermal, and catalytic properties of materials can change as the surface area to volume ratio increases at the nanoscale. At nanoscale sizes, many insulator materials begin to behave like conductors. The same applies to nanoscale dimensions, where we can see a variety of fascinating quantum and surface phenomena.
Physical and chemical properties of nanomaterials include size, shape, chemical composition, crystal structure, stability, surface area, surface energy, and other properties. The surfaces of nanomaterials become reactive with each other and with other systems as the surface-to-volume ratio increases. The pharmacological effects of nanomaterials are greatly influenced by their size. Nanomaterials can change their crystal structure upon interaction with water or other dispersing media. The aggregation state of nanomaterials depends on their size, composition, and surface charge. The magnetic, physicochemical, and psychokinetic properties of these materials are affected by surface coatings. Strong polar or covalent bonds or van der Waals forces are responsible for particle interaction at the nanoscale. Polyelectrolytes can be used to alter the surface characteristics of nanomaterials and their interactions with other substances and environments.
Classification of nanomaterials
Nanomaterials classification is necessary because of the enormous diversity of these tiny particles, which makes it impossible to place them under a single class. Diameter, size, surface area, morphology, dimensions, and manufacturing processes are all factors that may be used to categorise nanoparticles. However, nanomaterials are generally categorized based on their morphology, composition, uniformity, agglomeration, and also dimensionality.
Aspect ratio, sphericity, and flatness are taken into account in the morphology-based categorization of nanoparticles. But most research groups nanoparticles according to their aspect ratio. On the basis of aspect ratio, nanomaterials are divided into two categories which is high aspect ratio nanoparticles and low aspect ratio nanoparticles.
On the basis of low aspect ratio, nanoparticles are categorized as oval, cubical, prism-shaped, spherical, pillar-like and colloids. While, on the basis of high aspect ratio nanoparticles, nanoparticles can be divided into nanorods, nanohooks, nanohelices, nanostars, nanospring, and nanoplates.
Nanoparticles can be classified as zero-dimension (0D) where length, breadth and height are confined at single point like nanodots and nanoparticles, one-dimension (1D) where only one parameter length or breadth like nanowires and nanotubes, two dimension (2D) where only two parameter either length or breadth or height like very thin surface coatings, and three-dimension (3D) where it has all parameter of length, breadth, and height such as crystals.
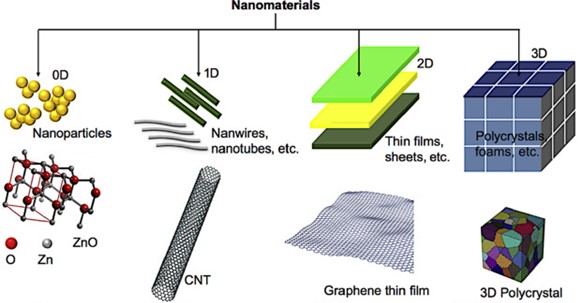
Figure 2. Schematic representation of 0D, 1D, 2D and 3D nanomaterials (Asha, 2020)
Nanoparticles can also be classified based on their composition. Some nanoparticles in nature are composed of only one type of substance, while others are composed of multiple different types of substances. Natural nanoparticles are often composites of different components. With today’s technology, it is simple to generate single, pure nanoparticles. Nanomaterials can be made from single substances such as antimony and bismuth, or combination of substances like hybrid nanozigzags built of nickel, silicon and titanium oxide.
- Uniformity and agglomeration state
Composition is not the only parameter that is used to distinguish and classify nanoparticles. Based on the uniformity and agglomeration state, nanoparticles can exist in dispersed aerosols and agglomerate state. In dispersion aerosols, they can exist in the form of colloids or suspensions. This classification is based on their electromagnetic properties such as magnetism, surface charge, and chemistry.
For example,magnetic nanoparticles tend to cluster, forming an agglomerate state, unless their surfaces are coated with a non magnetic material. In an agglomerate state, nanoparticles may behave as larger particles, depending on the size of the agglomerate. Hence, it is evident that nanoparticle agglomeration and size and surface reactivity, along with shape and size, must be taken into account when considering health and environmental regulations of new materials.
References
Elprocus. (2022). What are Nanomaterials – Classification and Its Properties. Retrieved from https://www.elprocus.com/nanomaterials-classifications-and-its-properties/
Gorai, D. S. (2019, August 9). Introduction to Nanomaterials and Nanotechnology. Retrieved from https://agc.ac.in/resources/Introduction_to_Nanomaterials_and_Nanotechnology.pdf
Jayvadan K. Patel, A. P. (2021, June 24). Introduction to Nanomaterials and Nanotechnology. Retrieved from https://link.springer.com/chapter/10.1007/978-3-030-50703-9_1
Rizwan, A. S. (2021). Chapter 3 - Types and classification of nanomaterials. Retrieved from https://www.sciencedirect.com/science/article/pii/B978012823823300001X
Nanowerk. (2022). What are Nanomaterials? Retrieved from https://www.nanowerk.com/what-are-nanomaterials.php
Vladimir Pokropivny, R. L. (2007). Introduction to Nanomaterials and Nanotechnology. Retrieved from https://www.researchgate.net/profile/Alex-Pokropivny/publication/299345334_Introduction_in_nanomaterials_and_nanotechnology/links/56f160e008ae1cb29a3d0c2d/Introduction-in-nanomaterials-and-nanotechnology.pdf
Zhang, L. (2019, June 27). Applications, Challenges, and Development of Nanomaterials and Nanotechnology. Retrieved from https://jcsp.org.pk/PublishedVersion/f7845589-394c-478e-aae3-4346169943f4Manuscript%20no%203,%20Final%20Galley%20 Proof%20of%2012199%20(Ling%20Zhang).pdf
Written by,
Nurintan binti Jamil, Internship Student, Nanomaterials Synthesis and Characterization Laboratory (NSCL), ION2 &
Dr. Mohd Hafizuddin Ab Ghani, Research Officer, Nanomaterials Synthesis and Characterization Laboratory (NSCL), ION2
Date of Input: 23/09/2022 | Updated: 06/05/2024 | roslina_ar
MEDIA SHARING